The weight of the system increases with the mass. An extensive property is a property that depends on the amount of matter in a sample.
For example the temperature of a system in thermal equilibrium is the same as the temperature of any part of it.

Is the weight of a system an extensive or intensive property?. It is a measure of strength. The mass of any substance depends on the size or quantity of matter. If the system is divided the temperature of each subsystem is identical.
An extensive property is dependent upon the amount of mass present or upon the size or extent of a system. The First Law of Thermodynamics. The extensive property is different from the intensive property and it depends on the amount of matter or the size of the system.
Show all show all steps. The properties cannot be used to define the identity of the system. The ratio of two extensive properties is an intensive property.
Weight of a system mass acceleration due to gravity. On the earths surface the weight of an object is equal to its mass. Heat shall not be confused with temperature.
What do you mean by extensive and intensive properties. Because of its value changes with changes of the mass. So weight of system depends on mass and mass is an extensive property.
The value of an extensive property varies directly with the mass. Temperature pressure specific volume and density are examples of intensive properties. Size of extensive properties.
Examples of extensive properties Weight. What is an Extensive Property. Is the weight of a system an extensive or intensive property.
The specific volume of water at 20 degrees is 0001002 m3 kg. Pressure can also be classically defined as Force per unit Area. Is the specific weight an.
So weight of a system is extensive property. Extensive properties are the microscopic properties of the systemmatter which are dependent on amount or quantity of a material present in the systemmatter. Similarly the volume also increases with the mass of the substances.
Temperature on the other hand is an intensive property as it is proportional to. Force is an extensive property because F ma and mass is extensive as it depends on the number of particles. Dividing in half a tank of air at a given T P and average molecular weight yields two smaller tanks containing air but the average molecular weight of the air in each new smaller tank is the same as it was intially in the one big tank.
Extensive properties are those whose values depend on the size or extent of the system. The heat capacity is directly proportional to the mass. It is the inverse magnitude to the density.
Heat is an extensive property and is proportional to the total energy of all atoms in an object. An intensive property is independent of the amount of mass and may vary from place to place within the system at any moment. The product of an intensive and an extensive property is extensive.
If 1 kg of rice is added the mass of rice will increase and it will become 11 kg. Mass total volume and energy are examples of extensive properties. Extensive properties are those whose values depend on the size or extent of the system.
These properties become half when the system is divided into two parts. The ratio of two extensive properties is an intensive property. The Second Law of Thermodynamics.
Intensive property is the one which is being defined at a point like temperature and pressure. These properties are proportional to the size or mass of the system. Walkthrough for Chapter 1 Problem 23P.
An Engineering Approach 8th physics. The specific weight of a system is defined as the weight per unit volume note that this definition violates the normal specific property-naming convention. Hence the weight is an extensive property.
It is the gravitational force that acts on an object. Average molecular weight is the ratio of mass to moles. Because of its value changes with changes of the mass.
So weight of a system is extensive property. Extensive properties like mass are dependent upon the amount of a substance while intensive properties like density are independent of quantity. What You Need To Know About Extensive Property.
Yes Heat is a property of matter. Walkthrough video for this problem. Extensive properties of any matter are those physical properties that depend on the mass of that matter.
An extensive property depends on the size of the system ie it is reported for the system as a whole. If we were to divide the system into smaller portions the weight of each portion would also be smaller. Although the volume is an extensive property the specific volume is an intensive property because it is the volume occupied by a unit of mass of a material.
Is the weight of a system an extensive or intensive property. Specific quantities are also referred to as intensive. For example cubic meters per kilogram.
Say 10 kg of rice. Because of its value changes with changes of the mass. Is the weight of a system an extensive or intensive property.
For example volume v of a system or number of moles of a given component. It is measured in units of volume by one unit of mass. Extensive properties are those whose values depend on the size or extent of the system.
So weight of a system is extensive property.

Difference Between Intensive And Extensive Properties Definition Examples And Differences Intense Properties Of Matter Physical Properties Of Matter
Can You Explain The Extensive And Intensive Property Of Thermodynamics Quora

Viscosity Comparison Chart Hapco Inc Viscosity Chart School Activities

Intensive Properties Extensive Properties Examples

Intensive Properties Extensive Properties Examples
Difference Between Intensive Property And Extensive Property

Properties Of Matter Physical Chemical Chemistrygod

Different Types Of Thermodynamic Systems Open System Closed System Isolated S Sponsored Paid Affiliate Thermodyn Thermodynamics System Biology Notes
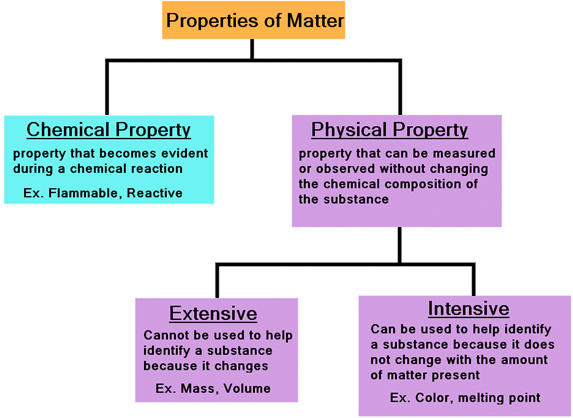
Properties Extensive And Intensive Texas Gateway

Lecture 1 Thermodynamic Properties Ppt Download

Green Roof Built Up System Green Roof System Green Roof Green Roof Benefits
Examples Of Intensive Extensive Properties Of Matter Video Lesson Transcript Study Com
Is Density Intensive Or Extensive Property Quora

Intensive Properties Extensive Properties Examples
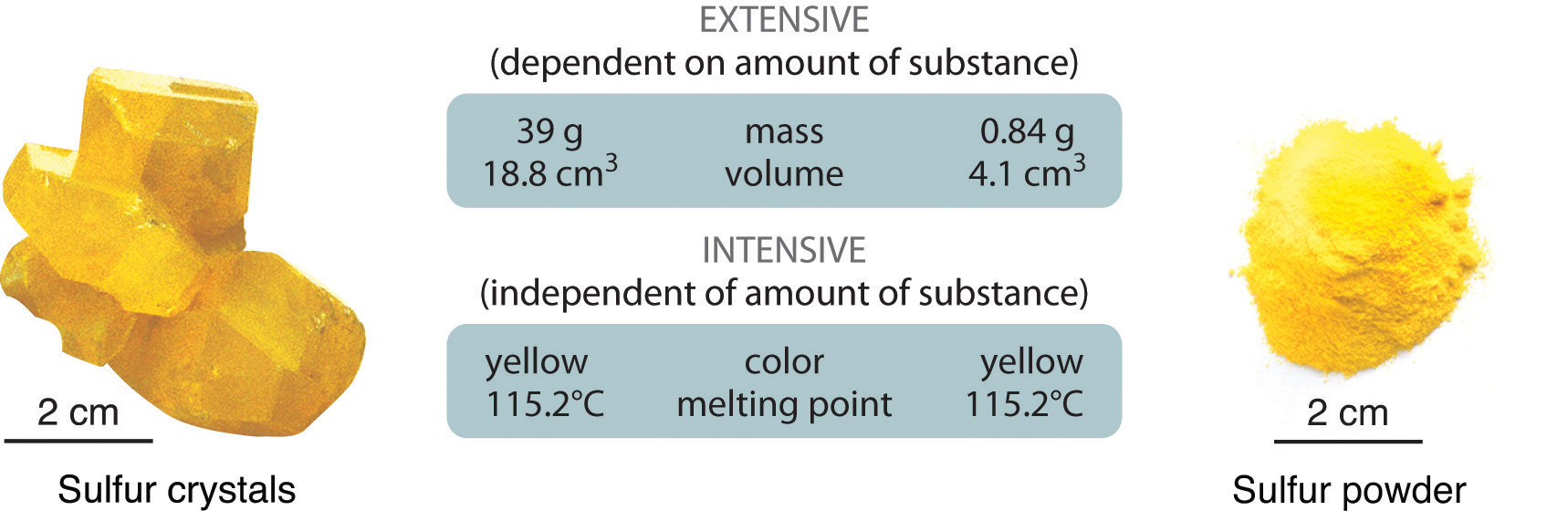
1 2 Properties Of Matter Chemistry Libretexts



